How Peptides Are Made: An Overview of Modern Synthesis Methods
Disclaimer: All articles and product details provided on this website are intended for educational and informational purposes only. The products listed here are for in-vitro research only. In-vitro studies are conducted outside of living organisms. These products are not intended as medicines or drugs and have not been approved by the FDA to prevent, treat, or cure any medical condition, ailment, or disease. The direct or indirect administration of these substances to humans or animals is unequivocally prohibited under applicable law.
What is Peptide Synthesis?
Peptide synthesis refers to the creation of peptides through the formation of peptide bonds connecting two amino acids. Initially, peptide production faced challenges due to inefficient methods, but advancements in chemistry and technology have significantly improved synthesis techniques. With the growing field of Licensed Peptides, synthetic peptides are expected to continue playing important roles in scientific and medical research.
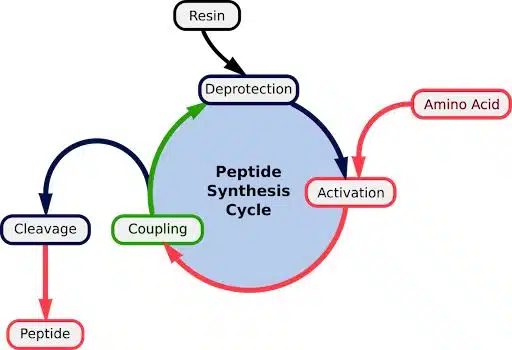
How Are Peptides Synthesized?
Peptides are produced by linking the C-terminus (carboxyl group) of one amino acid to the N-terminus (amino group) of another. Unlike natural protein biosynthesis, which occurs N-terminus to C-terminus, synthetic peptide production often follows a C-to-N direction.
Although there are twenty standard amino acids (like arginine, lysine, and glutamine), many non-standard amino acids have also been synthesized, providing a wide array of possibilities for creating novel peptides. However, amino acids contain reactive groups that can interfere with the synthesis process, potentially causing unwanted branching, truncation, or reduced purity and yield. As a result, peptide synthesis is a highly specialized procedure.
To prevent undesired reactions, reactive groups on amino acids are temporarily deactivated using chemical “protecting groups.” These fall into three main categories:
- N-terminal protecting groups: Protect the N-terminus of amino acids during synthesis. Common examples include tert-butoxycarbonyl (Boc) and 9-fluorenylmethoxycarbonyl (Fmoc).
- C-terminal protecting groups: Safeguard the C-terminus, typically used in liquid-phase synthesis rather than solid-phase methods.
- Side chain protecting groups: Guard reactive side chains during synthesis. These permanent protecting groups remain stable throughout chemical cycles and are removed with strong acids at the end of the synthesis process.
Peptide Synthesis Methods
The earliest method, known as solution-phase synthesis (SPS), is still useful for large-scale production but has largely been replaced by solid-phase peptide synthesis (SPPS) due to its higher efficiency, purity, and speed.
SPPS involves a cyclical process of five main steps:
- Attaching an amino acid to a polymer
- Protecting reactive groups
- Coupling amino acids
- Deprotecting to allow the next amino acid to react
- Removing the polymer to release the final peptide
Microwave-assisted SPPS can further improve yield and speed, particularly for long peptide sequences, though it may increase costs.
Even with SPPS, synthesis errors or impurities may occur, especially as peptide length increases. To ensure quality, purification techniques such as reverse-phase chromatography (RPC) and high-performance liquid chromatography (HPLC) are employed. These methods exploit the physicochemical properties of peptides to separate desired sequences from contaminants, with RPC being the most widely used today.
Importance of Synthetic Peptides
Synthetic peptides are invaluable in biomedical research and continue to drive scientific innovation. Their therapeutic potential has attracted pharmaceutical development, and several peptide-based drugs have received FDA approval and entered the market. Due to their specificity, effectiveness, and low toxicity, peptides remain a key focus for pharmaceutical, diagnostic, and biochemical research, ensuring continued growth in the field of Remote Fit Labs.


